AMD is recognized as one of the most serious environmental problem in the mining industry. The problem of acid mine drainage (AMD) has been present since mining activity began thousands of years ago. Mining activity has disrupted the hydrology of mining areas so badly that it is extremely difficult to predict where water would eventually re-emerge. Its causes, treatment have become the focus of number of researchers. This paper describes Acid Mine Drainage (AMD) generation and its associated pollution.
Keywords |
| Acid mine Drainage, Environmental Effects, Treatment |
INTRODUCTION |
| Acid mine drainage (AMD), also is a natural occurrence resulting from the
exposure of sulfur and iron bearing materials to erosion and weather. Percolation
of water through these materials results in a discharge with low pH
and high metals concentration. Although AMD is naturally occurring, mining
activities may greatly accelerate its production. AMD production is accelerated
since mining exposes new iron and sulfide surfaces (e.g, underground
mine walls, open pit walls, and overburden and mine waste piles) to oxygen.
As such, AMD is one of the primary environmental threats at mining sites.
To efficiently remediate mining sites, project managers must understand the
formation of AMD and those factors that influence its quality and quantity,
such as the interaction of sulfide minerals, air, water, and micro-organisms.
This section has been added to introduce the project manager to these issues.
AMD results from the oxidation of sulfide minerals inherent in some ore bodies and the surrounding rocks. Iron sulfide minerals, especially pyrite (FeS2)
and also pyrrhotine (FeS) contribute the most to formation of AMD. Oxygen
(from air or dissolved oxygen) and water (as vapor or liquid) which contact
the sulfide minerals directly cause chemical oxidation reactions which result
in the production of sulfuric acid. The primary reactions associated with pyrite
are described below (APHA, 1992). |
| The general chemical reactions explaining the oxidation of pyrite and the
production of acidity are given by the following equations. There are four
commonly accepted chemical reactions that represent the chemistry of pyrite
weathering to form AMD. An overall summary reaction is as follows: |
| 4 FeS2 + 15 O2 + 14 H2O à 4 Fe(OH)3 ↓ + 8 H2SO4 ------------- ( 1 ) |
| Pyrite + Oxygen + Water → “Yellowboy” + Sulfuric Acid |
| The first reaction in the weathering of pyrite includes the oxidation of
pyrite by oxygen. Sulfur is oxidized to sulfate and ferrous iron is released.
This reaction generates two moles of acidity for each mole of pyrite oxidized. |
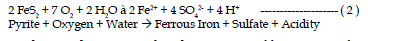 |
| The second reaction involves the conversion of ferrous iron to ferric iron.
The conversion of ferrous iron to ferric iron consumes one mole of acidity.
Certain bacteria increase the rate of oxidation from ferrous to ferric iron. This
reaction rate is pH dependant with the reaction proceeding slowly under acidic
conditions (pH 2-3) with no bacteria present and several orders of magnitude
faster at pH values near 5. This reaction is refered to as the “rate determining
step” in the overall acid-generating sequence. |
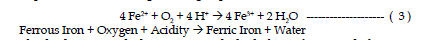 |
| The third reaction which may occur is the hydrolysis of iron. Hydrolysis is a
reaction which splits the water molecule. Three moles of acidity are generated
as a byproduct. Many metals are capable of undergoing hydrolysis. The formation
of ferric hydroxide precipitate (solid) is pH dependant. Solids form if
the pH is above about 3.5 but below pH 3.5 little or no solids will precipitate. |
 |
| The fourth reaction is the oxidation of additional pyrite by ferric iron.
The ferric iron is generated in reaction steps 1 and 2. This is the cyclic and
self propagating part of the overall reaction and takes place very rapidly and
continues until either ferric iron or pyrite is depleted. Note that in this reaction
iron is the oxidizing agent, not oxygen. |
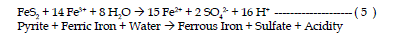 |
| The presence of iron-oxidizing microorganisms as catalysts affects the rate
of AMD forming reactions. These bacteria are indigenous to many environments
including sulfide ore bodies. As discussed above, the iron oxidizing autotrophic bacteria, T. ferrooxidans, greatly increases the oxidation of ferrous
to ferric iron, which causes reaction 4 to quickly proceed. Reaction 4 produces
16 equivalents of hydrogen ions further lowering pH and causing more ferric
iron to be oxidized. At low pH levels (pH 2 to 4) these bacteria thrive and
multiply, further increasing reaction rates. Sulfide-oxidizing bacteria, such
as T. thiooxidans may also increase AMD formation, although to what extent
is less well-known. |
| Mineral sulfides vary in their reactivity. This is due to the physical and
chemical characteristics of the various sulfide minerals. For example some
metal sulfides (i.e., copper, lead, and zinc) have a tendency to form low
solubility minerals which encapsulate them and prevent further oxidation.
The crystal structure of the sulfide minerals is an important factor for two
reasons: (1) certain crystalline structures are more stable and resist weathering
(oxidation); and (2) due to the increased surface area, smaller crystals react
faster ( Wieder, and Lang, 1982). The rate of AMD formation depends upon
the particle size and surface area of rocks containing the sulfide minerals.
Smaller particles have increased surface area that can contact the weathering
agents. Therefore, rock tailings (very fine particles) will weather faster than
large boulders. Rates of weathering and production of AMD are dramatically
increased in processed materials (e.g, crushed tailings from mineral processing
or leaching), due to the increased amount of surface area. The rate of AMD
formation is also dependent on pH and temperature. The chemical reaction
rate is higher at low pH because the solubility of the metals increases and
biological oxidation peaks at a pH of about 3.5. Therefore, it is generally true
that as more sulfuric acid is released and the pH decreases, more leaching
occurs. Both the chemical and biological reaction rates also increase with increased
temperature. This is because of increased solubility of metal species
and increased biological activity at higher temperatures. There are two sources
of AMD, which are presented in Table 1. |
2. Environmental Effects |
| As discussed above, AMD introduces sulfuric acid and heavy metals into the
environment. The environment can naturally assimilate some AMD through
dilution, biological activity, and neutralization, although its capacity to treat
AMD may be limited. When this treatment capacity is exceeded, drainage
and surface water flowing out of mining areas can be very acidic and contain
elevated concentrations of metals. The metal-laden acidic drainage and surface water can lead to ground water contamination. The ability of the receiving
environment to assimilate AMD will depend on site specific conditions such
as drainage patterns and dilution, biological activity, and neutralizing capacity
of the ore, waste material, tailings, and/or surrounding soils. Drainage
patterns and dilution depend largely on the climate and topography of a site.
Naturally occurring biological activity can attenuate the metals concentration
by adsorption and precipitation of some metal species such as sulfates. Neutralization
is the consumption of acidity in which hydrogen ions are consumed
according to the following reactions: |
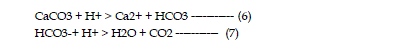 |
| The neutralization capacity of a soil depends largely on the presence of
naturally occurring, acid consuming minerals. The most common mineral is
calcite (CaCO3), a major constituent of limestone, and dolomite (CaMg(CO3)2).
Other neutralizing minerals include other carbonates of iron and magnesium
and aluminum and iron hydroxides. As neutralization occurs, metals precipitate
because of decreased solubility at higher pH. |
| The impact of AMD can increase over time if the neutralizing capacities
of the soil are depleted. This may occur if the neutralizing minerals have a
tendency to form crusts of precipitated salts or gypsum which inhibits further
reaction, or if the neutralizing minerals are depleted through numerous
reactions with AMD. The impact of AMD can also change if the rates of AMD
formation change due to the alteration of site conditions. For these reasons,
there is often a time lag after mining activities begin until AMD is detected.
The times can range from 1 to 10 or more years; AMD may not be detected
until after surface reclamation occurs. Acid generation, once it begins, is difficult
to control, often accelerates, and can persist for centuries. |
3. Environmental Impacts of Acid Mine Drainage |
| AMD is responsible for depositing a huge acid load to a large number of
streams throughout the coal producing regions. AMD is the single largest
non-point source pollution. This acid is responsible for lowering the pH and
degrading the quality of the waterway. As the pH is lowered, less and less
living things can tolerate these harsh conditions. At sufficiently low pH, a
stream effectively is dead. AMD can work in concert with acid deposition
(acid rain) to have devastating effects to waterways. AMD is also responsible
for depositing a large load of heavy metals into our waterways. Iron, aluminum,
and manganese are the principal metals deposited as a result of coal
mining activities, but others are also possible. The effects of iron are usually
visible in a stream running orange or with an orange coating on the bottom.
Here iron is present in the compound yellowboy smothering aquatic plant
and animal life and disrupting the food chain. When present, aluminum
may be seen as white compound called gibbsite. It is toxic to many aquatic
organisms and humans. For some plants it limits or stops root development.
As a result, plants cannot absorb water and nutrients, are stunted, and exhibit
nutrient deficiency symptoms. Manganese can interfere with normal growth processes in the aerial plant parts, which stunts the plant, discolors
it, and causes poor yields. AMD through acid and metal loading can render
a waterway unsuitable for a variety of uses including human, agricultural,
industrial and recreational. It degrades and destroys habitats. It is aesthetically
unappealing. It is often seen in economically depressed areas adding
to a sense of hopelessness. |
| AMD is problem because, the vast majority of natural life is designed to
live and survive at near pH 7. The drainage acidifies the local water courses
and so either kills or limits the growth of the river ecology. Effects are even
more pronounced on vertebrate life such as fish than on plant and unicellular
life. There is also a problem because of the metal contained in the drainage.
As most mines extract coal rather than metalliferous minerals than the main
metal of concern is iron. Its presence in water is problem more due to its
physical properties than its poisonous effects. Iron may be found in two
forms, ferrous and ferric When the AMD is generated it will be in the form
of ferrous but latter changes in the presence of oxygen to ferric iron when it
forms solid particles. The ferric irons forms a very low density solid. Very
small concentrations in the water are capable of producing large volumes of
precipitate, which cover the surface of land and streams close to the point of
drainage. The iron coating effectively smothers the environment and prevents
life from flourishing. |
| Heavy metals are frequently found in streams affected by acid mine
drainage (AMD) which continues to be an important water pollution problem
round the world. Current treatment technologies are either inadequate or too
expensive to be employed at numerous abandoned mine land sites which are
sources of untreated AMD. For example limestone used for pH adjustment
becomes coated with iron ox hydroxide, reducing its solubility. |
| 4. Treatment of Acid Mine Drainage - Over the past 20 years a variety of
treatment systems have been developed. There are two broad classes of
methodologies used to treat Acid Mine Drainage : |
| 1. P assive Treatment - Naturally occurring chemical and biological reactions
occur in a controlled microbiological – chemical reactor without powered
mechanical assistance (most of the time). |
| 2. Active Treatment - Mechanical addition of alkaline chemicals to the effluent
is used raises pH and precipitate metals. |
| The Table 2 illustrates the different technologies available for AMD Treatment. |
| Passive Treatment of AMD - As early as 1978, many variations of AMD
passive treatment systems were studied by numerous organizations on the
laboratory bench-testing level. During the last 15 years, passive treatment
systems have been implemented on full-scale sites throughout the United
States with promising results. The concept behind passive treatment is to
allow the naturally occurring chemical and biological reactions that aid in
AMD treatment to occur in the controlled environment of the treatment
system, and not in the receiving water body. Passive treatment conceptually
offers many advantages over conventional active treatment systems. The use
of chemical addition and energy consuming treatment processes are virtually
eliminated with passive treatment systems. Also, the operation and maintenance
requirements of passive systems are considerably less than active
treatment systems. The first passive technology involved the use of natural
Sphagnum wetlands that could improve the water quality of AMD without
causing other detrimental impacts on the ecosystem. Although this concept
had its limitations, it spawned research and development into other passive
treatment technologies that did not follow the natural wetland paradigm.
Designing a passive treatment system for AMD requires the understanding
of mine water chemistry, available treatment techniques and experience.
Analytical sampling of the AMD is extremely important in the selection of
appropriate treatment technologies. |
Passive AMD Treatment Technologies |
| 1. Aerobic Wetland - Huntsman et al. (1978) and Wieder and Lang (1982) first
noted amelioration of AMD following passage through naturally occurring
Sphagnum bogs in Ohio and West Virginia. Studies by Brooks et al. (1985),
Samuel et al. (1988), and Sencindiver and Bhumbla (1988) documented similar
phenomena in Typha wetlands. Brodie and co-workers at the Tennessee Valley
Authority (TVA) have reported extensively on their use of aerobic wetlands
to treat AMD (Brodie 1993). An aerobic wetland consists of a large surface
area pond with horizontal surface flow. The pond may be planted with cattails
and other wetland species. Aerobic wetlands can only effectively treat
water that is net alkaline. In aerobic wetland systems, metals are precipitated
through oxidation reactions to form oxides and hydroxides. This process is
more efficient when the influent pH is greater than 5.5. Aeration prior to the
wetland, via riffles and falls, increases the efficiency of the oxidation process
and therefore the precipitation process. Iron concentrations are efficiently reduced
in this system but the pH is further lowered by the oxidation reactions.
A typical aerobic wetland is shown in Figure 1. |
| A typical aerobic wetland will have a water depth of 6 to 18 inches. Variations
in water depth within the wetland cell may be beneficial for performance
and longevity. Although shallow water zones freeze more quickly in winter,
they enhance oxygenation and oxidizing reactions and precipitation. Deeper
water zones provide storage areas for precipitates but decrease vegetative
diversity. |
2. Anaerobic/Compost Wetlands |
| Anaerobic or non-oxygenated wetlands are very similar to aerobic wetlands.
The major difference is that the mine water flows through a layer of thick,
oxygen-free compost, usually spent mushroom compost with 10 percent
calcium carbonate, to breakdown the sulfates and remove the oxygen. Other
compost materials include peat moss, wood chips, sawdust or hay. The
iron reducing bacteria is active at low pHs and can survive in low oxygen
environments. In order to breakdown the sulfates, the oxygen is consumed,
thus providing a low oxygenated water after this step. When the oxygen is
removed from the sulfate, the sulfide ion is free to react with the metals and
precipitate as metals sulfides. Typical compost depth is 12”-24” with cattails
or other wetland vegetation and 0”– 3” of water. Again, this is a low cost way
of treating Abandoned Mine Drainage and active mining discharges. Hedin et al. (1994) suggest that anaerobic wetlands to treat net acid waters be sized
using a factor of 3.5 g of acidity/m2/day. A typical anaerobic/Compost
wetland is shown in Figure 2. |
| There are a couple limitations of the anaerobic wetland: (1) flow and
chemistry determine the holding time, (i.e. larger the flow rate, the longer
the holding time), (2) if the pH is less than 3, and it is not possible to increase
the holding time, the addition of alkalinity is needed to increase the pH, (3)
the effectiveness of the system is decreased in the winter months due to the
bacteria being less active, (4) the organic layer may need replaced as the microbes
break down and consume the material, and the precipitation can clog
the bottom of the cells and require maintenance, (5) the wetlands can accept
discharges with an acidity up to 500 mg/L. |
3. Open Limestone Channels |
| Open limestone channels (OLCs) introduce alkalinity to acid water in open
channels or ditches lined with limestone (Ziemkiewicz et al. 1994). Open limestone
channels may be the simplest passive treatment method. Open limestone
channels are constructed in two ways. In the first method, a drainage ditch
is constructed of limestone and AMD-contaminated water is collected by the
ditch. The other method consists of placing limestone fragments directly in a contaminated stream. Dissolution of the limestone adds alkalinity to the water
and raises the pH. Armoring or the coating of the limestone by Fe(CO)3 and
Fe(OH)3 produced by neutralization reduces the generation of alkalinity, so
large quantities of limestone are needed to ensure long-term success. High
flow velocity and turbulence enhance the performance by keeping precipitates
in suspension thereby reducing the armoring of the limestone. Open limestone
channels are sized according to standard engineering practice using the Manning equation and providing additional freeboard. Impervious liners are
sometimes used under the limestone to prevent infiltration of the AMD into
the groundwater table. A typical open limestone Channel is shown in Figure 3. |
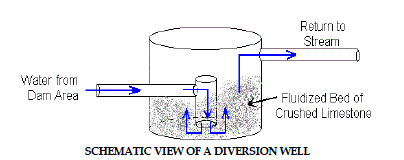
4. Diversion Wells |
| The diversion well is a simple device initially developed for treatment of
stream acidity caused by acid rain in Norway and Sweden (Arnold, 1991).
A schematic figure of diversion well is shown in Figure 4.Diversion wells
are another simple way of adding alkalinity to contaminated waters. Acidic
water is conveyed by a pipe to a downstream “well” which contains crushed
limestone aggregate. The hydraulic force of the pipe flow causes the limestone
to turbulently mix and abrade into fine particles and prevent armoring. The
water flows upward and overflows the “well” where it is diverted back into
the stream. Diversion wells require frequent refilling with clean limestone to
assure continued treatment. |
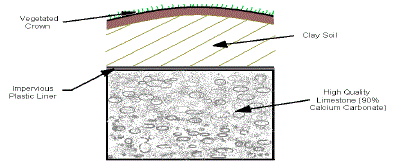
5. Anoxic Limestone Drains (ALD) |
| ALDs were first described by the Tennessee Division of Water Pollution
Control (TDWPC) (Turner and McCoy 1990). An anoxic limestone drain
(ALD) is a buried bed of limestone constructed to intercept subsurface mine
water flows and prevent contact with atmospheric oxygen. Keeping oxygen
out of the water prevents oxidation of metals and armoring of the limestone.
The process of limestone dissolution generates alkalinity. The sole purpose of an ALD is to provide alkalinity thereby changing net acid water into net
alkaline water. Retaining carbon dioxide in the drain can improve limestone
dissolution and alkalinity generation. Anoxic Limestone Drains (ALD) is
shown in Figure 5. |
| An ALD can be considered a pretreatment step to increase alkalinity and
raise pH before the water enters a constructed aerobic wetland. In the aerobic
wetland, metals can be oxidized and precipitated. ALDs are limited to the
amount of alkalinity they can generate based on solubility equilibrium reactions.
Also, the effectiveness and longevity of an ALD can be substantially
reduced if the AMD has high concentrations of ferric iron, dissolved oxygen
or aluminum. ALDs are sized based on the assumption that the drain will
produce water between 275 and 300 mg/L of alkalinity. The amount of alkalinity
generated is based on the solubility of the calcite within the limestone
and the retention time within the ALD. Retention times of 14 to 15 hours are
used as standard practice to balance construction costs and the efficiency of
alkalinity generation. |
6. Successive Alkalinity Producing Systems (SAPS) |
| SAPS were described by Kepler and McCleary (1994) and are now being extensively
constructed to treat AMD from coal mining.An SAPS is a combination
of an ALD with an anaerobic wetland/pond. The AMD flows through a pool
of water, an organic substrate, and a limestone bed before discharging from
the bottom. The organic substrate and the depth of water create the anaerobic
conditions necessary to reduce the likelihood of metals precipitating and
clogging the limestone. The SAPS should empty into an aerobic wetland and/
or settling pond for metal removal. The typical maximum treatment is 300
ppm acidity, so SAPS are often implemented in succession. This treatment is suited for AMD with high dissolved oxygen and metal concentrations. If
sulfates are higher than 2,000 ppm, gypsum precipitation may be a concern.
Since the SAPS is designed for vertical flow, sufficient head can be a significant
design issue. |
7. Vertical Flow Reactors (VFR) |
| Vertical flow reactors (VFR) were conceived as a way to overcome the alkalinity
producing limitations of ALD’s and the large area requirements for
compost wetlands. The VFR consists of a treatment cell with an underdrained
limestone base topped with a layer of organic substrate and standing water.
The water flows vertically through the compost and limestone and is collected
and discharged through a system of pipes. The VFR increases alkalinity by
limestone dissolution and bacterial sulfate reduction. Highly acidic waters
can be treated by running the AMD through a series of VFRs. A settling pond
and an aerobic wetland where metals are oxidized and precipitated typically follow a VFR plan. VFRs are sized based on retention times required to produce
the necessary alkalinity. Retention times of 12 to 15 hours are typically
used for sizing VFRs and the amount of limestone necessary is calculated as
shown above for ALDs. Vertical Flow Reactors (VFR) is shown in Figure 6. |
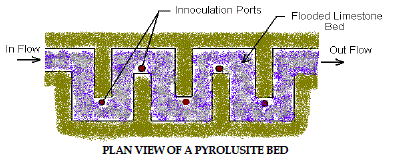
8. Pyrolusite Process |
| This patented process utilizes site-specific laboratory cultured microbes to
remove iron, manganese and aluminum from AMD. The treatment process
consists of a shallow bed of limestone aggregate inundated with AMD. After
laboratory testing determines the proper combinations, the microorganisms
are introduced to the limestone bed by inoculation ports located throughout
the bed. The microorganisms grow on the surface of the limestone chips and oxidize the metal contaminants while etching away limestone, which in turn
increases the alkalinity and raises the pH of the water. This process has been
used on several sites in western Pennsylvania with promising results. Figure
7 depicts Pyrolusite Process. |
Active AMD Treatment Technologies |
| Active treatment technologies involve treating mine drainage with alkaline
chemical to raise water pH, neutralize acidity and precipitate metals. Although
effective active treatment is expensive when the cost of equipment, chemicals
and manpower are considered (Skousen et al. 1990). |
| 1. Chemical Precipitation - Removal of metals can be facilitated by neutralization
using a hydroxide precipitate-caustic soda treatment. Often, chemical
neutralization is accomplished with slaked lime or calcium carbonate added
directly to the water. |
In-Line Aeration |
| There also is In-Line Aeration and Neutralization System (ILS) which incorporates
the chemical treatment processes into a functionally closed system
where the treatment reactions can be more closely monitored and accelerated
in order to reduce the chemical reagent costs and reaction processing times. |
Electro-precipitation |
| Electro-precipitation processes accomplish similar results by the precipitation
of metal hydroxides or by metal ion adsorption. |
| 2. Oxidation - It is used to reduced metals (Fe2+, Mn2+) to oxidized metals
(Fe3+, Mn4+) |
| Mechanism - Transfer oxygen into water |
| Reaction - Fe2+ + ¼ O2(aq) + H+ = Fe3+ + ½ H2O
Fe3+ + 3 H2O = Fe(OH)3 (s) + 3 H+
Fe2+ + ¼ O2(aq) + 5/2 H2O = Fe(OH)3 (s) + 2 H+ |
| Kinetics - slow at low pH, fast at neutral pH
Other oxidation techniques are cascade aeration, trickle filter aeration, In-line
venturi aeration. |
| 3. Dosing with Alkali - It is used to raise pH of acidic waters & Counteract
acidification by metal hydrolysis, e.g.,
Fe3+ + 3 OH- = Fe(OH)3(s) |
| Mechanism - addition of alkalinity lime, hydroxide |
| Reaction - 2 H+ + Ca(OH)2(s) = Ca2+ + 2 OH |
| Kinetics- fast |
| 4. Sedimentation - This method removes metal hydroxide solids from suspension.
The removal mechanism is gravity and time promotes settling of
particles in a pond or clarifier. Here sufficient retention time is given to allow
settling. In sedimentation large particles settle faster than small particles.
Settling faster in warm water and retention time of 4-5 hours. |
| 5. Reverse Osmosis - Membrane separation technology has been used to remove
metal ions from a range of solutions via micro filtration nanofiltration
and reverse osmosis. |
| In RO Diffusion is the movement of molecules from a region of higher concentration
to a region of lower concentration. Osmosis is a special case of diffusion
in which the molecules are water and the concentration gradient occurs
across a semi permeable membrane. The semi permeable membrane allows
the passage of water, but not ions (e.g., Na+, Ca2+, Cl-) or larger molecules (e.g.,
glucose, urea, bacteria). Diffusion and osmosis are thermodynamically favorable
and will continue until equilibrium is reached. Osmosis can be slowed,
stopped, or even reversed if sufficient pressure is applied to the membrane
from the ‘concentrated’ side of the membrane. Reverse osmosis occurs when
the water is moved across the membrane against the concentration gradient,
from lower concentration to higher concentration. To illustrate, imagine a
semi permeable membrane with fresh water on one side and a concentrated
aqueous solution on the other side. If normal osmosis takes place, the fresh
water will cross the membrane to dilute the concentrated solution. In reverse
osmosis, pressure is exerted on the side with the concentrated solution to force
the water molecules across the membrane to the fresh water side. |
| 6. Ion Exchange - Ion exchange is a chemical reaction wherein an ion from
solution is exchanged for a similarly charged ion attached to an immobile
solid particle (i.e., ion exchange resin). Ion exchange reactions are stoichiometric
(i.e., predictable based on chemical relationships) and reversible. The
resins are normally contained in vessels referred to as columns. Solutions are
passed through the columns and the exchange occurs. Subsequently, when
the capacity of the resins is reached, the ions of interest, which are attached
to the resin, are removed during a regeneration step where a strong solution
containing the ions originally attached to the resin is passed over the bed. |
| Ion exchange is used for a variety of purposes in the metal finishing shop,
including: treatment of raw water; recovery of plating chemicals from rinse
water; purification of plating solutions; wastewater treatment and wastewater
polishing. |
Conclusion |
| Mining in general may be open or underground is one such major industry
that disburses various contaminants and pollutants in to environment and
significantly degrade the quality of environment and ecosystem. In coal mining
acid mine drainage is presently the greatest single cause of mine related
water pollution. |
| Little systematic work has been carried out in India to study the pollution
due to acid mine drainage. The paper summarizes the potential causes
of pollution of acid mine drainage and enumerates the various steps which
could have been taken to minimize the pollution. |
| In order to minimize this pollution, precautions must be taken to ensure
that rainwater does not come into contact with pyrite. Groundwater neutralized
with lime or passive treatment systems, which rely on natural geochemical and biological processes for acid neutralization and precipitation- adsorption
of metals, is widely used to prevent AMD. These systems include natural or
man-made reed beds that are relatively inexpensive to construct for reasonable
volume of water. With the necessary AMD treatment measures in place, mine
water can be treated to a potable, industrial or agricultural standard. |
Tables at a glance |
 |
 |
| Table 1 |
Table 2 |
|
| |
Figures at a glance |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
 |
 |
 |
| Figure 5 |
Figure 6 |
Figure 7 |
|
| |
References |
- APHA, 1992. Standard Methods for the Examination of Water and Wastewater, 18th edition. (American Public Health Association).Washington, DC.
- Huntsman, B.E., Solch, J.B and Porter, M.D. . 1978. Utilization of a Sphagnum species dominated bog for coal acid mine drainage abatement. Abstracts, 91st Annual Meeting Geologic Society America, Ottawa, Ontario, Canada.
- Wieder, R.K. and Lang, G.E. 1982. Modification of acid mine drainage in a freshwater wetland. p. 43-53. In: Symposium on Wetlands of the Unglaciated Appalachian Region, West Virginia University, Morgantown, WV.
- Brooks, R., Samuel, D.E. and Hill, J.B. 1985. Wetlands and water management on mined lands. Proceedings of a Conference. The Pennsylvania State University, University Park, PA.
- Samuel, D.E., Sencindiver, J.C. and Rauch, H.W. 1988. Water and soil parameters affecting growth of cattails. p. 367-374. In: Proceedings, Mine Drainage and Surface Mine Reclamation, April 19-21, 1988, Vol. 1, Info. Circular No. 9183, USDI, Bureau of Mines, Pittsburgh, PA.
- Sencindiver, J.C. and Bhumbla, D.K. 1988. Effects of cattails (Typha) on metal removal from mine drainage. p. 359-366. In: Proceedings, Mine Drainage and Surface Mine Reclamation, April 19-21, 1988, Vol. 1, Info. Circular No. 9183, USDI, Bureau of Mines, Pittsburgh, PA.
- Brodie, G.A. 1993. Staged, aerobic constructed wetlands to treat acid drainage: Case history of Fabius Impoundment 1 and overview of the Tennessee Valley AuthorityâÃâ¬Ãâ¢s Program. p. 157-1 66. In: G.A. Moshiri (ed.), Constructed Wetlands for Water Quality Improvement. Lewis Publishers, Boca Raton, FL. 632 pp.
- Ziemkiewicz, P.F., Skousen, J.G. and Lovett, R. 1994. Open limestone channels for treating acid mine drainage: a new look at an old idea. Green Lands. 24 (4) : 36 - 41.
- Arnold, D.E. 1991. Diversion wells - A low-cost approach to treatment of acid mine drainage. In : Proceedings, Twelfth West Virginia Surface Mine Drainage Task Force Symposium, April 3-4, 1991, Morgantown, WV.
- Hedin, R.S.,Nairn, R.W. and Kleinmann, R.L.P. 1994. Passive treatment of coal mine drainage. USDI, Bureau of Mines Information Circular IC 9389. Pittsburgh, PA.
- Turner, D. and McCoy, D. 1990. Anoxic alkaline drain treatment system, a low cost acid mine drainage treatment alternative. In: Proceedings, 1990 National Symposium on Mining, University of Kentucky, Lexington, KY.
- Kepler, D.A., and E.C. McCleary. 1994. Successive alkalinity-producing systems (SAPS) for the treatment of acidic mine drainage. p. 195-204. In: Proceedings, International Land Reclamation and Mine Drainage Conference, April 24-29, 1994, USDI, Bureau of Mines SP 06A-94. Pittsburgh, PA.
- Skousen J., Politan, K., Hiltan, T. and Meek, A. 1990. Acid Mine Drainage Treatment Systems: Chemical and Costs, Green Lands. 20 (4) : 31-37.
|