Keywords
|
| Sulfite oxidase, Molybdenum, Spinaceae oleraceae, Sulfur dioxide |
INTRODUCTION
|
| Sulfite oxidase is a vital enzyme catalyzing the oxidation of sulfite to sulfate. The terminal reaction in the oxidative degradation of the sulfur containing amino acids cysteine and methionine is catalysed by this mitochondrial enzyme. It belongs to the molybdenum cofactor (Mo-Co) containing family of enzymes. |
| It has long been known that the rare transition element molybdenum is an essential micronutrient for plants, animals and microorganisms (Bortels et al. 1930). Molybdenum and tungsten are the only second and third row transition metals that are required for the growth of at least some organisms; molybdenum in particular is an essential trace element for most living systems, including microorganisms, plants, and animals (Stiefel, 1993). X-ray crystallographic analysis of Mo-enzymes reveal that the cofactor is not located on the surface of the protein, but it is buried deeply within the interior of the enzyme and a tunnel-like structure makes it accessible to the appropriate substrates (Kisker et al. 1997). Once Mo-co is liberated from the holoenzyme, it loses Mo and undergoes rapid and irreversible loss of function due to oxidation (Rajagopalan and Johnson, 1992). The demolybdo-forms of Mo-enzymes are catalytically inactive (Kisker et al. 1997). Elevated amounts of the Mo-antagonist, tungsten, were shown to inhibit the activity of Mo-enzymes by replacing Mo as a ligand of MPT (Wray and Filner, 1970; Kisker et al. 1997). |
| The best characterized sulfite oxidizing enzymes from the sulfite oxidase family are those from avain and mammalian sources (Rajagopalan, 1980), that are homodimers containing heme b and molybdenum coordinated via an MPTtype molybdenum pterin cofactor and a conserved cysteine residue. However, the occurrence of sulfite oxidase in plants was a matter of slight controversy for a long time, because this enzyme would catalyze a reaction that counteracts sulfate assimilation. In animals, however, it is a well-studied enzyme involved in the degradation of sulfur-amino acids (Eilers et al. 2000). However, various scientists using different technical procedures observed its presence in plants (Ganai et al. 1997, Eilers et al. 2000) but the presence of molybdenum as cofactor of sulfite oxidase has not been demonstrated as yet. In order to deal with the same objective Spinaceae oleraceae plant was selected to check for the role of molybdenum in the activity of sulfite oxidase. |
MATERIALS AND METHODS
|
Plant Material
|
| Twenty-four earthen pots, each of 15cm diameter were selected for sowing of Spinaceae oleraceae seeds. Pots were divided into four sections with six pots in each section. Seeds were equally divided in each section. Pots were kept under natural environmental conditions. Plants in each section were daily treated with 10,100, 1000 ppm of sodium tungstate solution respectively. Plants grown in fourth section (control) were treated with 200mL of water. |
| Sampling of Tissue - Leaves from all four sections were collected separately, cut into small pieces, weighed for fresh and dry weight and kept for preparation of homogenate. |
| Dry Weight Determination - The weighed samples were kept for drying at 105°C in an oven for two hours and then at 70° C till a constant weight was attained. |
| Preparation of Homogenate - A 10% (W\V) homogenate was prepared. Weighed sample of tissue was homogenized in chilled water using pestle and mortar. The homogenate was filtered through eight layered muslin cloth. The muslin filtered homogenate was centrifuged in cold at 500 × g for 10 minutes. The supernatant was subsequently used for enzyme assay and other estimations. |
| Sulfite oxidase assay - Sulfite oxidase was assayed at pH 7.8, essentially by the method described by Macleod et al. (1961), using sodium sulfite as the substrate. The activity of the enzyme was indicated by decrease in absorbance of potassium ferricyanide at 420 nm that was used as an indicator substrate in the coupled assay. |
| Estimation of proteins - Proteins were estimated by the method of Lowry et al (1951). |
| Estimation of amino acids - Estimation of total amino acids was carried out by the method of Lee and Takahashi (1960). |
| Generation and estimation of sulfur dioxide - Aqueous sulfur dioxide was generated by reducing hot concentrated |
 |
Sulfur dioxide was estimated according to West and Gaeke (1956).
|
| Treatment of leaf discs from each section with aqueous sulfur dioxide and preparation of homogenate - Leaf discs of 1 cm diameter from each section (10,100,1000ppm sodium tungstate treated) were treated with 100ppm of aqueous sulfur dioxide for four hours in petridishes (15 × 20 mm) under light which was provided by a 100 W electric bulb. Treatment conditions were kept similar for each section. After 4 hours leaf discs were separated, washed, patted dry, weighed and homogenized. |
RESULTS
|
Effect of sodium tungstate treatment on the activity of sulfite oxidase
|
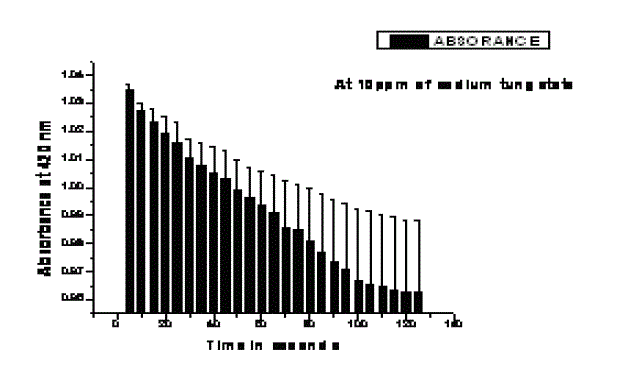
| The change in absorbance of sulfite oxidase is shown in Fig. 1. The activity was measured in terms of decrease in absorbance at 420nm at 15 seconds interval for 4 minutes. Fig.2 shows change in absorbance of sulfite oxidase in sample treated with 10ppm of sodium tungstate. Sulfite oxidase activity got decreased to 89.85% (Table 1). Fig.3 shows change in absorbance of sample treated with 100ppm of sodium tungstate where activity got decreased to 98.1% (Table 1). No sulfite oxidase activity was seen in plant treated with 1000ppm of sodium tungstate. Tungsten treatment decreased the activity of sulfite oxidase to 100 %. |
Effect of sodium tungstate treatment on protein content of Spinaceae oleraceae
|
| No significant decrease in the concentration of proteins was observed on treatment with sodium tungstate (Table 2). |
Effect of SO2 exposure on proteins of Spinaceae oleraceae treated with 100 ppm sodium tungstate
|
| The effect of SO2 exposure on proteins of Spinaceae oleraceae treated with 100 ppm sodium tungstate is shown in Table 3. The plant shows a decrease of 73.3% in protein concentration on exposure to 100ppm sulfur dioxide. However, a decrease of only 44.8% is observed in tungsten untreated plants on exposure to the same concentration of sulfur dioxide. |
Effect of SO2 exposure on amino acids of Spinaceae oleraceae treated with 100 ppm sodium tungstate
|
| Table 4 shows decrease of 23.9% in amino acid concentration in Spinaceae oleraceae plant on exposure to 100ppm of sulfur dioxide, while a decrease of 58.6% is observed in plants treated with sodium tungstate on exposure to same concentration of sulfur dioxide. |
Effect of SO2 exposure on photosynthetic pigments of Spinaceae oleraceae treated with 100 ppm sodium tungstate
|
| Spinaceae oleraceae shows a decrease of 29.9% in chlorophyll content on exposure of 100ppm of SO2 while a significant decrease of 60.1% was observed in plants treated with 100ppm of sodium tungstate on exposure to same concentration of sulfur dioxide. Pheophytins show a decrease of 37.5% when the plants were exposed to 100ppm of SO2 while a significant decrease of 71.85% was found in plants treated with 100ppm of sodium tungstate on exposure to same concentration of sulfur dioxide (Table 5). |
DISCUSSION
|
| For many years tungsten was considered to be a biological antagonist of molybdenum and was used for study of the properties and functions of molybdenum in Mo-enzymes (L’vov, 1989). This was due to the fact that tungsten is able to replace molybdenum in Mo-enzymes, forming catalytically inactive (or possessing very low activity) analogs (L’vov et al. 2001). Certain microorganisms can also utilize tungsten in a similar fashion (Kisker, 1997). |
| Sulfite oxidase which catalyses the physiologically vital oxidation of sulfite to sulfate in animals, plants and bacteria, belongs to the molybdenum cofactor (Mo-Co) containing family of enzymes. The best characterized sulfite oxidizing enzymes from the sulfite oxidase family are those from avain and mammalian sources (Rajgopalan, 1980), that are homodimers containing heme b and molybdenum coordinated via an MPT-type molybdenum pterin cofactor and a conserved cysteine residue. However the occurrence of sulfite oxidase in plants was a matter of slight controversy for a long time, because this enzyme would catalyze a reaction that counteracts sulfate assimilation. However various scientists using different technical procedures observed its presence in plants (Ganai 1997 and Eilers et al. 2000). |
| Molybdenum as the second-row transition metal required by most living organisms forms the catalytically active center of all Mo enzymes, except nitrogenase (Schwarz et al. 1990) and few species that do not require molybdenum use tungsten, which lies immediately below molybdenum in the periodic table. |
| Because of their unique chemical versatility and similarity in structures, it is taken up by various enzymes in a similar manner as that for tungsten (Kisker et al. 1997). Measurable sulfite oxidase activity was observed in Spinacea oleraceae grown under normal environmental conditions in absence of any external stress. While same plant under similar environmental conditions when treated with sodium tungstate showed no sulfite oxidase activity. Plants grown in presence of high concentration of tungsten, take up tungsten instead of molybdenum in its enzyme. The tungsten cofactor incorporated sulfite oxidase did not show any sulfite oxidizing activity. Therefore, our findings are in agreement with the above facts. |
| Sulfur dioxide when dissolved in water, dissociates to form sulfite, bisulfite and other ionic species. Both sulfite and bisulfite have been shown to be deleterious to plants (Winner et al. 1985). It is reported that multiple effects of sulfur dioxide and sulfite on metabolism include pigment destruction (Syanrhyeichyk and Syanrhyeichyk, 1995), interference with the activity of various enzymes (Niewiadomska et al. 1997) and destruction of various amino acids like methionine and tryptophan during aerobic oxidation of sulfite (Yang, 1973). Cowling and Koziol, 1978 observed a significant reduction in glycine and serine contents, as a result of sulfur dioxide exposure. But plants can overcome these phytotoxic effects by readily converting bisulfite and sulfite into less toxic sulfate ions by the activity of sulfite oxidase (Babich and Stotzky, 1980; Huber et al. 1987). |
| Plants made deficient in sulfite oxidase on treatment with sodium tungstate showed greater decrease in chlorophyll, amino acid and protein content, on treatment with aqueous sulfur dioxide. Since the replacement of molybdenum by tungsten inactivates sulfite oxidase, detoxification of sulfite and bisulfite ions is hampered, which ultimately leads to increased destruction of different components. These findings are in consistence with the conclusion that sulfite oxidase is instrumental in counteracting the toxic effects of sulfur dioxide and molybdenum acts as a cofactor in sulfite oxidase enzyme in Spinacea oleraceae. |
Tables at a glance
|
 |
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
Table 5 |
|
| |
Figures at a glance
|
 |
| Figure 1 |
|
| |
References
|
- Babich, H. and Stotzky, G.1980. Environmental factors that influence the toxicity of heavy metals and gaseous pollutants to microorganisms. CRC critical reviews in Microbiology. 8 : 99-145.
- Bortels. H. 1930. Molybdenum alsKatalysatorbei der biologischenStickstoffbindung. ArchivfürMikrobiologie. 1 : 333-342.
- Cowling, D.W. and Koziol, M.J. 1978. Growth of rye-grass (Loliumpernne L.) exposed to sulfur dioxide. I: Effect on photosynthesis and respiration. Journal of Experimental Botany. 29 : 1029-1036.
- Eilers, T.H., Christina, R., Tim, N., Jorg, B., Henner, S., Gunter, H., Robert, M., Ralf, R. 2000 The missing link in S-metabolism: Sulfite Oxidase in Plants. Plant Biology. 2000 Abs # 562 G
- anai, B.A., Masood, A. and Baig, M.A. 1997. Isolation, purification and partial characterization of sulfite oxidase from MalvaSylvestris. Photochemistry. 54 : 779- 780.
- Hinze, H. and Holzer, H. 1986. Analysis of the energy metabolism after incubation of Saccharomyces cerevisiae with sulfite or nitrite. Arch. Microbiol. 145 : 27-31.
- Huber, K., Esterbauer, E., Jager, H.J. and Grill, D. 1987. Detection of sulfites in plants. Environmental Pollution. 46 : 127-136.
- Kisker, C., Schindelin, H. and Rees, D.C. 1997. Molybdenum-cofactor-containing enzymes: structure and mechanism. Annual Review of Biochemistry 66, 233-267.
- Kisker, C., Schindelin, H., Baas, D., Retey, J., Meckenstock, R.U. and Kroneck, P.M. 1998. A structural comparison of molybdenum cofactor-containing enzymes. FEMS Microbiology Reviews. 22 : 503-521.
- L’vov, N.P. 1989. Molybdenum in Nitrogen Assimilation in Plants and Microorganisms, 43rd A. N.Bach Memorial Lecture (in Russia), Nauka, Moscow.
- L’vov, N.P., Nosikov, A.N. and Antipov, A.N. 2001. Tungsten – containing enzymes Biochemistry (Moscow). 67 (2) : 196-200.
- Leblanc, et al. 1972. The epiphyte vegatation of Populasbalsanofisa and its significance as an air pollution indicator in Sudbury, Ontario. Canada. J . Bol. 50 : 519.
- Lee, Y.P. and Takahashi, T. 1966. An improved colorimetric determination of amino acids with the use of ninhydrin. Anal. Biochem. 14 : 71-77.
- Lowry, O.H., Rosebrough, N. J., Farr, A. L. and Randall, R. J. 1951. Protein measurement with the Folin - Phenol reagent. J. Biol. Chem. 193 : 265-275.
- MacLeod, R.M., Farkas, W., Fridovitch, I. and Handler, P. 1961. Purfication and properties of hepatic sulfite oxidase. J. Biol.Chem. 236 (1961) : 1841-1846.
- Rajagopalan, K.V. 1980. Sulfite oxidase, p. 241-271. In : M. P. Coughlan (ed.), Molybdenum and Molybdenum-Containing Enzymes. Pergamon, Oxford, United Kingdom.
- Rajagopalan, K.V. and Johnson, J.L. 1992. The pterin molybdenum cofactors. Journal of Biological Chemistry. 267 : 10199-10202.
- Richard, F.E. 1965. Microscopic analysis of plants and fungi under environmental stress from air pollution. Arch. Environ. Contam. Toxicol. 18 : 277-284.
- Schwarz, G, Stallmeyer, B., Kuper, J., Eilers, T. and Ralf, R. 1990. Mendel Molybdenum insertion: The last step of molybdenum cofactor biosynthesis. Weekly Magazine, Department of Botany, Technical University of Braunschweig, D- 38023 Braunschweig, Germany
- Stiefel, E.I., Coucouvanis, D. and Newton, N.W. 1993. Molybdenum Cofactors and Model Systems, Vol. 535. Washington,DC: Am. Chem. Soc. 387 pp.
- Syarhyeichyk, S.A. and Syarhyeichyk, A.A. 1995. The influence of gaseous industrial toxicants on primary photosynthetic radiation of coniferous species under Belarus conditions. Cent Bot. Gard. Acad. Sci. Belarus Minsk. Belarus pp5-9.
- Thompson, et al. 1965. Effect of peroxyacetyl nitration on ultrastructure of chloroplast. Bot gaz. 126 : 66-69.
- Winner, E.W., Monney, A.H. and Goldstin, A.R. 1985. Sulfur Dioxide and Vegetation. Physiology, Ecology and Policy Issue. Stanford Univ. press, Stanford California.
- West, P.W. and Gaeke, G.C. 1956. Fixation of sulfur dioxide as disulfitomercurate (II) and subsequent colorimetric estimation. Annal Chem. 28 : 1816-1819.
- Wray, J.L. and Filner, P. 1970. Structural and functional relationships of enzyme activities induced by nitrate in barley. Biochemical Journal. 119 : 715-725.
- Yang, S.F. 1973. Destruction of tryptophan during the aerobic oxidation of sulfite ions. Environ. Res. 6 : 395-402.
|