Keywords
|
| Polyoxyethylated octyl phenol, CMC, Surface excess (Γ) property |
INTRODUCTION
|
| The surfactant molecules can organize themselves into aggregates when dissolved into aqueous medium. The micelle formation takes place above critical micelle concentration (cmc), below which surfactant molecules are present as monomers (Tanford, 1980 and Myers1992). The functions and properties of surfactant systems depend on their structural type, concentration and compositions, besides, temperature, pressure, pH, solvent and additives. There has been, recently, upsurge in commercial application of nonionic surfactants and the progress in basic research (Schick, 1982). Keeping in mind the properties of nonionic surfactants (Sulthana et al. 2000 and Koshy and Rakshit, 1991), we stretched our work, in continuation of our interest in the properties of polyoxyethylated octyl phenol to explore the interfacial and micellization properties of polyoxyethylated octyl phenol at different pHs to understand how acidity/alkalinity affect the behavior of the surfactant in aqueous solution (Dave et al. 2005). The effects of change of pH and temperatures of polyoxyethylene (10) lauryl ether [C12E10: CH3 (CH2)11(CH2CH2O)10OH] have been investigated (Sharma and Rakshit, 2004). The aggregation behavior of some polyoxyethylated alkyl ethers(Sahoo, 2002) and anionic surfactants (Sahoo et al. 2002) in aqueous medium using fluorescence spectroscopy. The effect of pH on other surfactants like dodecyldimethylamine oxide (DDAO) (Maeda, 1996), cationic hexadecyltrimethyl ammonium bromide (HTAB) (Behrends and Herranann, 2000) and amphoteric-anionic N,N-dimethyl N-lauroyl lysine (DMLL)-sodiumdodecyl sulphate (SDS) (Abe et al. 1989) have also been investigated. The results of pH on dimethyl dodecyl amine oxide (DDAO) showed that it behaves as nonionic at pH >7, as cationic (DDHA+) at pH £ 3 and as a nonionic-cationic mixture between pH 3 and 7 have been reported by Herrann (Herrmann, 1964). |
| In the present investigation, the effect of pH on surface excess (Γ), minimum area per molecule (Amin),surface pressure (Πcmc) and thermodynamics of micellization and adsorption of polyoxyethylated octyl phenol [C8H17-O(CH-2CH2O)30-H] at the air-water interface at different temperatures have been reported. The study of the effect of pH on micellization is important because of their ability to adsorb selectively on silica surface (Fu, 1987 and Patryka et al. 1984 ) and applications in processes such as detergency (Stensby, 1981),cosmetics (Idson, 1985), enhanced oil recovery ( Fu, 1987) etc. The surface activity of this surfactant has been correlated with its structure. |
Methods
|
Reagents
|
| Polyoxyethylated octyl phenol surfactant was procured from Nikko Chemicals, Japan under the commercial name OP-30. This surfactant was used as received. The absence of minimum in the surface tension vs. concentration plot confirmed the high purity (> 99 %) of this surfactant (Okano et al. 2000). |
| Sodium chloride (Ranbaxy Fine Chemicals Ltd., India) for maintaining constant ionic strength was used without any further purification. The solutions of surfactants in triply distilled water having conductivity 2-3 γ Scm-1 containing 0.01 M NaCl were prepared afresh for surface tension measurements. A digital pH meter of Systronics-335 was used, following calibration using buffer solutions of pH 4.0,7.0 and 9.2 obtained from S.D. Fine Chemicals Ltd., Borisar, India. In the working solution,0.01 M HCl and NaOH were used to adjust acidic and alkaline pH respectively |
Apparatus
|
| The critical micelle concentration (cmc) was determined by the surface tension (γ) measurements using a du-Nouy ring tensiometer (S.C.Dey and Co. Calcutta, India) measurements were carried out at temperatures 303, 308, 313 and 318 K. The temperatures were maintained within ± 0.1 K by circulating thermostated water through a jacketed vessel containing the solution. The surface tension measurements were done as reported by Dave et al. 2005. The measured surface tension values were plotted as a function of the logarithm of surfactant concentration and the critical micelle concentration (cmc) was estimated from the break point in the resulting curve (Song and Rosen, 1996). The cmc does not change with the addition of 0.01 M NaCl. |
RESULTS AND DISCUSSION
|
| Surface tension is a reliable and excellent method for determination of cmc (Menger and Keiper, 2000). The cmc values of polyoxyethylated octyl phenol [C8H17-O(CH-2CH2O)30-H] at various pHs and at different temperatures are depicted in Table1. It can be seen that at constant temperature, cmc increases with the pH of the solution, low pH conducive for the micellization of the surfactant. It is quite apparent from Table 1 that cmc values of polyoxyethylated octyl phenol decrease with an increase in temperature at all pH, a typical characteristic of nonionic surfactant. |
| From Table 1, it is emerged that with decreasing [H+] or lowering of temperature increases the cmc. Both H+ and OH- can make hydrogen bonds with water molecules and thereby promote water structure, which is also promoted by the hydrophobic group of the surfactant molecule. On the other hand, increasing the temperature, the oxyethylene groups get dehydrated with decrease in hydrophilicity or increase in hydrophobicity causing lowering of cmc. |
| The standard free energy of micellization is associated to cmc by the following expression (Rosen,1988). |
 |
| the values are given in Table 2. |
| The ΔGm0 values are all negative which manifests that the formation of micelle is a spontaneous process. Moreover, with increasing temperature the free energy of micellization is rather more negative revealing comparative spontaneity of the micellization process as temperature increases. |
| The ΔSm0 was enumerated from the slop of the ΔGm0 vs. T plots. The ΔHm0 had been computed from the expression (Attwood et al. 1985). |
 |
| The free energy of micellization (ΔGm0 ) and the enthalpy of micellization (ΔHm0 ) are both positive denoting that the micellization process is entropy driven process. High entropy changes are generally associated with a phase change. The pseudophase micellar model is, therefore, opted over the mass action model. Furthermore, the poly(oxyethylene) moiety in [C8H17-O(CH-2CH2O)30-H] chain can be protonated at low pH, leads to increase in entropy of micellization, making it positively charged to behave as a pseudo ionic surfactant. At alkaline pH, decreasing entropy of micellization due to deprotonation, making it to remain nonionic. |
| The enthalpies and entropies of micellization process, as presented in Table 2,were plotted against each other and an excellent linear compensation effect was observed as suggested by Lumry and rajender (Lumry et al. 1970).. The slop of the line is the compensation temperature has been found to be 292 K, close to the expected values between 270 and 294 K in aqueous medium (Lumry et al. 1970).. The existence of such enthalpy-entropy compensation effect was also observed earlier for many physicochemical processes including monolayer and micellization (Sharma and Rakshit, 2004; Lumry, and Rajendra, 1970; Lumry and Rajendra, 1970; Singh and Singh, 1984 and Leffler et al. 1963). |
| In order to calculate the amount of surfactant adsorbed per unit area at air-water interface, these surface pressure (Πcmc= γ0- γcmc , where γ0 and gcmc are the surface tension of solvent and the surface tension of surfactant solution at cmc respectively) is fitted to the Gibbs adsorption equation. Assuming the adsorption density of water to be zero, the Gibbs adsorption equation (Defay et al. 1966) may be written as: |
 |
| Where, dΠ= the change in the surface pressure in the solution, |
| Γ = the adsorption density of the surfactant, |
| dμ = the change in the chemical potential of the surfactant, |
| R = universal gas constant, |
| T = absolute temperature, |
| C = concentration of the surfactant in aqueous solution. |
| Since the surfactant solutions are dilute, the activity is easily replaced by concentration. Eq. 3 can be written as; |
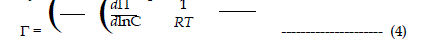 |
| Manimum adsorption density is calculated by limiting the concentration in the above equation to cmc of the surfactant |
 |
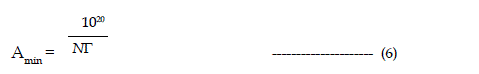 |
| Where N is the Avogadro number. |
| It is seen from Table 3, the surface excess increases with increase in temperature due to dehydration of the surfactant molecule. This is ascribed to compact adsorption at the air-water interface, two forces are operating; the hydrophobic interaction of the alkyl chain and the cross sectional area of the poly(oxyethylene) unit (as the surfactant anchors at the interface through hydrogen bonding between poly (oxyethylene) unit and the water molecule as shown in Fig. 1. This is also supported from the fluorescence behavior of pyrene in the presence of polyoxyethylated alkyl phenol (Sahoo et al. 2002 & Sahoo and Misra, 2001). The effect of temperature on Γat different pH is a complex phenomenon. This is influenced by the interaction of H+ and OHions with water as well as with the surfactant. The nature of interaction is complicated. |
| The Amin values of polyoxyethylated octyl phenol [C8H17-O(CH-2CH2O)30-H] at cmc are exhibited in Table 3. The magnitudes of Amin are of the order of 1.0 × 102 (A°2) or less, indicating that the surface is a close packed one which refers that the orientation of the surfactant molecules is perpendicular to the surface31. The value of free energy of adsorption at air-water interface (ΔG0ad) has been obtained using the expression (Rakshit and Narayan, 1986). |
 |
| In Table 4, the thermodynamic parameters of adsorption viz. G0ad, ΔH0adand ΔS0ad of polyoxyethylated octyl phenol at the air-solution interface at various pHs are shown. The standard entropy (ΔS0ad) and (ΔH0ad) of adsorption have been calculated from the slope of the reasonably linear ΔG0ad vs. T plot. The ΔH0ad has been obtained from the thermodynamic relation (Eq.2). The free energy of micellization, ΔG0m was less negative than the free energy of adsorption, ΔG0ad values at air-water interface at all temperatures, suggesting that when a micelle is formed, work has to be done to transfer the surfactant molecules in the monomeric form at the surface to the micellar stage through the aqueous medium. |
CONCLUSION
|
| Like the micellization process, the adsorption at the air-water interface has been found to be also endothermic. The endothermic character of micellization and adsorption are specific to the surfactant, the additive and the temperature (Sulthana et al. 1996; Koshy and Rakshit, 1991; Rakshit and Narayan, 1986 and Del Rio et al. 1995).The compensation temperature has been found to be 275K, some what different from 292 K obtained for micellization phenomenon, but within the expected range for aqueous systems (270-294 K) ( Lumry and Rajendra, 1970). |
ACKNOWLEDGEMENT
|
| The authors thank Mr. N.N. Patel, for assisting experimental work and preparing manuscript of this paper and Dr. R. M.Patel, Head, Department of Chemistry, S.P.University,V.V.Nagar, for providing facility to do this work. |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
|
| |
Figures at a glance
|
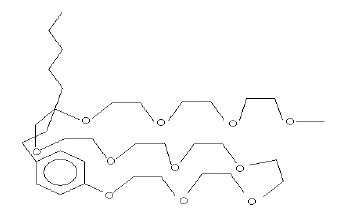 |
| Figure 1 |
|
| |
References
|
- Abe, M.K., Kato , K. and Ogino, J. 1989. Coll Interface Sci. 127 : 328.
- Attwood, D. and Florence, A. 1985. Surfactant Systems; Their Chemistry, Pharmacy and Biology, Chapman and Hall London. 95. Behrends, T. and Herranann, R. Coll Surf, 2000. 162 : 15.
- Clint, J.H. 1992. Surfactant Aggregation (Blakie, London), 16. Dave, P.N., Parmar, N.J. and Sait, S.S. 2005. Res J Chem Environ. 9 (4) : 55.
- Del Rio, J.M., Sarmiento, F.P. and Mosquera, V. 1995. Langmuir. 11 : 1511.
- Defay, R., Pregonine, I., Bellemans, A. and Evertt, D.H. 1966. Surface Tension and Adsorption. Longmans, London. Fu, E. 1987. Adsorption of anionic-nonionic surfactant mixtures on oxide minerals, Ph.D. thesis, Columbia University, New York. Herrmann, K.W. 1964. J PhysChem, 68 : 1540.
- Idson, B. 1985. Surfactants in Cosmetics, edited by Martin M. Reiger, Marcel Dekker, New York. Koshy, L., Rakshit, A.K. 1991. Bull ChemSoc, Japan. 64 : 2610.
- Lawrence, S.A., Pilc, J.A., Readman, J.R. and Sermon, P.A. 1987. J. Chem. Soc .Commun. 1035. Lumry, P. and Rajendra, S.1970. Biopolymer. 9 : 1125.
- Leffler, J.E. and Grunwald, E. 1963. Rates and Equilibria of Organic Reactions, Willey, New York. Myers, D. 1992. Surfactant Science and Technology, 2nd ed., VCH, New York,Chap.3. Maeda, H. 1996. Coll Surf. 109 : 263
- . Menger, F.M. and Keiper, J.S. 2000. AngewChem Int. 39 : 1906.
- Okano, T., Tamura, T., Abe, Y., Tasuchida, T., Lee, S. and Sogthar, G. 2000. Langmuir. 16 : 1508. Patryka, S., Zaini, S., Lindheimer, M. and Brun, B. 1984. Coll Surf. 12 : 255.
- Rosen, M.J. 1989. Surfactants and Interfacial Phenomenon, 2nd ed., Willey, New York, Chap. 3. Rosen, M.J. 1988. Surfactants and Interfacial Phenomenon, Wiley, New York.
- Rosen, M.J., Cohen, W., Dahanyake, M., Hua, X.Y. 1982. J Phys Chem. 86 : 541. Rakshit, A.K. and Narayan, S. 1986. Indian J Chem. 25A : 951.
- Schick, M.J. 1982. Nonionic Surfactant, Marcel Dekker, New York, 13 : 2.
- Sulthana, S.B., Rao, P.V.C., Bhat, S.G.T., Nakana, T.Y., Sugihara, G. and Rakshit, A.K. 2000. Langmuir. 16 : 980. Sulthana, S.B., Bhat, S.G.T and Rakshit, A.K. 1996. Coll Surf, 111 : 57.
- Sharma , K.S. and Rakshit, A.K. 2004. Indian J Chem. 43A : 265.
- Sahoo, L., Misra, P.K. and Somasundaran, P. 2002.Indian J Chem. 41A : 1402. Sahoo, L ., Sarangi, J. and Misra, P.K. 2002. Bull ChemSoc (Japan). 75 : 12.
- Stensby, P.S. 1981. Detergency: Theory and Test Methods. Part III, edited by W. G. Cutler and R.C. Davis, Marcel Dekker, New York, 766. Song, L.D. and Rosen, M.J. 1996. Langmuir. 12 : 1149.
- Singh, H.N., Singh, R.P. and Singh, S. 1984. Indian J. Chem. 23A : 677.
- Sahoo, L. and Misra, P.K. 2001. SambalpurUniv J Sci Tech. 13 : 18.
- Tanford, C. 1980. The Hydrophobic Effect-Formation of Micelles and Biological Membranes. 2nd ed., Willey, New York, Chap. 6.
|